Abstract
Background
The combination of venetoclax and 5-azacytidine (5-AZA) for older and unfit patients with newly diagnosed AML has led to significant improvements in remission rates and survival compared to 5-AZA alone. We previously reported encouraging results with a low-intensity backbone of CLAD/LDAC alternating with a hypomethylating agent (HMA) for older patients with AML observing better outcomes than historical experience with HMA alone. We hypothesized that the addition of venetoclax to the CLAD/LDAC alternating with HMA backbone may further improve outcomes for an expanded cohort of older patients with newly diagnosed AML.
Methods
This is a phase II study investigating the combination of venetoclax with CLAD/LDAC alternating with AZA in older (age ≥ 60y) or unfit patients with newly diagnosed AML (excluding APL, CBF). The primary objective was composite complete response rate (CRc; CR+CRi); secondary endpoints were overall survival (OS), disease-free survival (DFS), overall response rate (ORR), and toxicity.
Induction was cladribine 5 mg/m 2 IV over 30 minutes on D1-5 and araC 20mg SQ BID on D1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m 2 IV on D1-3 and araC 20 mg SQ BID on D1-10 alternating with 2 cycles of AZA 75 mg/m 2 on D1-7, for up to 18 cycles. Venetoclax 400 mg was added on days 1-21 of each cycle with dose adjustments for concomitant CYP3A inhibitors. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
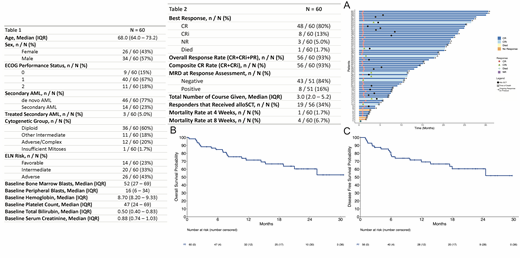
A total of 60 patients were treated on study with a median age was 68 years (IQR 64 - 73, range: 57 - 84); 22 (37%) patients were ≥ 70 yrs and 1 pt < 60 yrs who was unfit for intensive chemotherapy was enrolled. 14 (23%) patients had secondary AML (sAML). 36 (60%) had diploid cytogenetics with 12 (20%) patients having adverse cytogenetics at enrollment. By European Leukemia Network (ELN) risk, 23%, 33%, and 43% were favorable, intermediate, and adverse risk, respectively. The most commonly mutated genes were NPM1 in 21 patients (33%), DNMT3A in 20 (32%), TET2 in 18 (30%), SRSF2 in 15 (25%), NRAS in 12 (20%), IDH2 in 11 (18%), RUNX1 in 11 (18%), and ASXL1 in 9 (15%). TP53 was mutated in 4 (7%) patients. Baseline characteristics are summarized in table 1.
Among 60 evaluable patients the CRc rate was 93%. Best response was CR in 48 (80%), CRi in 8 (13%), no response in 3 (5%), and death in 1 (2%) patient. Responses are summarized in Figure A. In responding patients with a bone marrow sample evaluable for assessment of measurable residual disease (MRD), 43/51 (84%) were negative for MRD at response assessment. Among patients with sAML, with adverse karyotype, or ELN adverse risk the CR/CRi rate was 86% (64%/21%), 83% (58%/25%), and 96% (81%/15%) respectively. 19 (34%) responders received a subsequent allogeneic stem cell transplantation. Early mortality was low with one patient (2%) dying within 4 weeks and four patients (7%) dying with in 8 weeks. Responses are summarized in table 2.
The most frequent grade 3/4 non-heme adverse events were febrile neutropenia (n=10), pneumonia (n=5), atrial fibrillation (n=2), and allergic reaction (n=2). One patient developed grade 4 tumor lysis syndrome.
With a median follow up of 20.4 months, the median duration of response (DOR) is not reached (95% CI: 18 - NE months). Estimated 12- and 24-month DOR are 69.2% (95% CI: 57.5 - 83.1%) and 60.5% (95% CI: 47.7 - 76.8%), respectively. Median OS is not yet reached (95% CI: 21 - NE months). Estimated 12- and 24-month OS are 71.5% (95% CI: 60.5 - 84.5%) and 60.4% (95% CI: 47.7 - 76.6%), respectively (figure B). The estimated 12-month OS for patients aged <70 years and ≥70 years was 75% and 73%, respectively. Median DFS is not yet reached (95% CI: 18.0 - NE months). Estimated 12- and 24-month DFS are 69.2% (95% CI: 57.5 - 83.1%) and 60.5% (95% CI: 47.7 - 76.6%), respectively (figure C).
Conclusion
CLAD/LDAC plus venetoclax alternating with AZA plus venetoclax is an effective, lower-intensity regimen that is well tolerated among older patients (≥ 60 years) with newly diagnosed AML, producing high response rates with durable MRD negative remissions. The rates of overall and disease-free survival are encouraging in this cohort of older AML patients with comparable efficacy in patients ≥70 as in patients <70 years old. Further study of this non-anthracycline containing backbone in younger patients unfit for intensive chemotherapy, as well as comparisons to standard frontline therapies are warranted.
Kantarjian: AbbVie: Honoraria, Research Funding; Taiho Pharmaceutical Canada: Honoraria; Ascentage: Research Funding; BMS: Research Funding; Aptitude Health: Honoraria; Daiichi-Sankyo: Research Funding; Astellas Health: Honoraria; Pfizer: Honoraria, Research Funding; Immunogen: Research Funding; Novartis: Honoraria, Research Funding; Jazz: Research Funding; Amgen: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria; KAHR Medical Ltd: Honoraria; Astra Zeneca: Honoraria; Precision Biosciences: Honoraria; NOVA Research: Honoraria. Borthakur: Ryvu: Research Funding; ArgenX: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Astex: Research Funding; Protagonist: Consultancy; Takeda: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Pemmaraju: Affymetrix: Consultancy, Research Funding; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Sager Strong Foundation: Other; LFB Biotechnologies: Consultancy; Daiichi Sankyo, Inc.: Other, Research Funding; Springer Science + Business Media: Other; Aptitude Health: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Incyte: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; CareDx, Inc.: Consultancy; Clearview Healthcare Partners: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; MustangBio: Consultancy, Other; Plexxicon: Other, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Roche Diagnostics: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Celgene Corporation: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. DiNardo: Forma: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding. Sasaki: Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Daver: Bristol Myers Squibb: Consultancy, Research Funding; Novimmune: Research Funding; Glycomimetics: Research Funding; ImmunoGen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Hanmi: Research Funding; Abbvie: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Sevier: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Issa: Syndax Pharmaceuticals: Research Funding; Novartis: Consultancy, Research Funding; Kura Oncology: Consultancy, Research Funding. Short: Novartis: Honoraria; NGMBio: Consultancy; Takeda Oncology: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; Astellas: Research Funding; Amgen: Consultancy, Honoraria. Jain: Incyte: Research Funding; Genentech: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Beigene: Honoraria; Janssen: Honoraria; Servier: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Pharmacyclics: Research Funding; Aprea Therapeutics: Research Funding; TG Therapeutics: Honoraria; Precision Biosciences: Honoraria, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Ferrajoli: AstraZeneca: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board ; BeiGene: Other: Advisory Board, Research Funding. Takahashi: Novartis: Consultancy; Celgene/BMS: Consultancy; Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy. Popat: Bayer: Research Funding; Abbvie: Research Funding; Novartis: Research Funding; Incyte: Research Funding. Andreeff: Senti-Bio: Consultancy; Karyopharm: Research Funding; ONO Pharmaceuticals: Research Funding; Aptose: Consultancy; Medicxi: Consultancy; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company; Breast Cancer Research Foundation: Research Funding; Amgen: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Glycomimetics: Consultancy; AstraZeneca: Research Funding; Oxford Biomedica UK: Research Funding; Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy. Konopleva: AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Cellectis: Other: grant support; AstraZeneca: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Sanofi: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Forty Seven: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Ascentage: Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Agios: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; KisoJi: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights. Ravandi: AstraZeneca: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria; Agios: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding; Xencor: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Prelude: Research Funding; Astex: Honoraria, Research Funding; Taiho: Honoraria, Research Funding. Kadia: Liberum: Consultancy; Jazz: Consultancy; Novartis: Consultancy; Genfleet: Other; AstraZeneca: Other; Sanofi-Aventis: Consultancy; Ascentage: Other; Astellas: Other; Cellonkos: Other; Pulmotech: Other; Pfizer: Consultancy, Other; Amgen: Other: Grant/research support; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Genentech: Consultancy, Other: Grant/research support; Dalichi Sankyo: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal